Graphite Processing: From Raw Material to High-Value Applications
 Essow
Essow
 May 27, 2024
May 27, 2024
 1523
1523
If you want to know more details about equipment, solutions, etc, please click the button below for free consultation, or leave your requirements!
Graphite is a naturally occurring form of carbon that is known for its unique properties, including its high electrical conductivity, lubricity, and heat resistance. It is a mineral that is found in metamorphic rocks, and is commonly used in various industrial applications.
Graphite has a layered structure, with each layer consisting of carbon atoms arranged in a hexagonal lattice. These layers can easily slide over each other, giving graphite its lubricating properties. This makes graphite an ideal material for use in lubricants, such as in the automotive and machinery industries.
Graphite is also a good conductor of electricity, which makes it an important material in the production of electrodes for batteries, fuel cells, and electrical components. Its high thermal stability and resistance to high temperatures make it suitable for use in high-temperature applications, such as in the production of crucibles and refractory materials.
In addition to its industrial applications, graphite is also used in various consumer products, such as pencils, sporting goods, and electronic devices. It is valued for its smooth writing ability and its ability to conduct electricity in electronic devices.
Overall, graphite is a versatile material with a wide range of applications due to its unique properties and characteristics.
01 Mining and Extraction
BackGraphite is typically found in metamorphic rocks such as schist and gneiss, as well as in igneous rocks like granite. The mineral is usually mined using open pit or underground methods, depending on the depth and location of the graphite deposit. Once the ore is extracted from the ground, it is transported to a processing plant for further refinement.
At the processing plant, the graphite ore is crushed and ground into fine particles to liberate the graphite flakes from the surrounding rock. The ore is then subjected to a series of flotation processes to separate the graphite from the gangue minerals, such as quartz and feldspar. Flotation is a common method used in mineral processing to concentrate valuable minerals by selectively attaching them to air bubbles and separating them from the unwanted gangue minerals.
Graphite extraction and processing involve a series of steps to transform raw graphite ore into usable forms with desired properties. The process typically includes mining, ore preparation, beneficiation, purification, and further processing. Here is a closer look at each stage:
Graphite beneficiation aims to separate graphite from other minerals and impurities through various techniques. The most common beneficiation method is flotation, which utilizes the differences in the hydrophobic and hydrophilic properties of graphite and the accompanying gangue minerals. In the flotation process, the crushed and ground ore is mixed with water and specific reagents that selectively attach to the graphite particles, allowing them to float while the gangue minerals sink. The floated graphite concentrate is then collected and further processed.
The flotation process involves the following steps:
1. Grinding and Conditioning
The graphite ore is crushed and ground to a suitable size for flotation. It is then mixed with water and various reagents, such as collectors and frothers, in conditioning tanks. The purpose of conditioning is to create a suitable environment for the attachment of the collector to the graphite particles.
2. Flotation Cell
The conditioned graphite ore slurry is introduced into a flotation cell, which is typically a large tank equipped with impellers or agitators. The agitation promotes the formation of air bubbles in the slurry.

(Flotation Cells)
3. Collector Addition
Collectors, such as hydrophobic organic compounds, are added to the flotation cell. These collectors selectively attach to the graphite particles, making them hydrophobic and enabling their attachment to the air bubbles.
4. Air Bubble Attachment
As the impellers or agitators generate air bubbles in the flotation cell, the hydrophobic graphite particles attach to the bubbles, forming graphite-bubble aggregates or froth.
5. Froth Removal'The froth containing the graphite-bubble aggregates rises to the surface of the flotation cell, forming a concentrate. This concentrate, enriched in graphite, is skimmed off and collected as the final product.
6. Gangue Disposal
The gangue minerals, which are hydrophilic and do not attach to the air bubbles, remain in the flotation cell as tailings and are discharged.
The success of graphite flotation relies on the proper selection and dosage of collectors, pH control, and flotation cell conditions to optimize the recovery and purity of the graphite concentrate. Factors such as the ore's mineralogy, particle size distribution, and the presence of impurities influence the flotation process.
The flotation method is particularly effective in separating graphite from complex ore types and achieving high-grade concentrate with improved graphite content. It helps remove impurities, such as silicates, sulfides, and other non-graphitic carbon, which can negatively impact the quality and performance of the final graphite product.
Graphite flotation plays a critical role in the production of high-quality graphite concentrates, which are further processed and refined to meet the specific requirements of various applications, including lithium-ion batteries, lubricants, refractories, and electrical components.
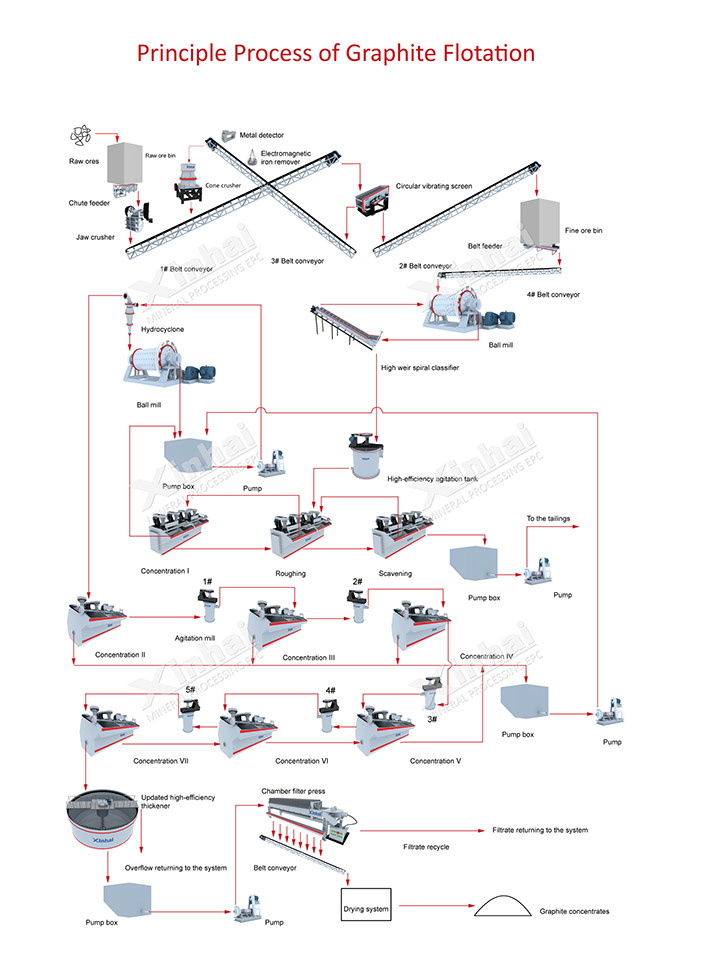
(Principle Process for Graphite Flotation)
02 Purification and Refining
BackAfter the graphite has been separated from the gangue minerals, it undergoes a series of purification steps to remove impurities and increase its carbon content. The most common method used for graphite purification is chemical treatment, where the ore is treated with acids or alkalis to remove contaminants and increase the purity of the graphite.
Another method used for graphite purification is thermal treatment, where the ore is heated to high temperatures in the presence of an inert gas to remove impurities and increase the carbon content of the graphite. This process, known as thermal exfoliation, can also be used to produce expanded graphite, which is a lightweight and highly absorbent material used in a variety of applications, including as a fire retardant and thermal insulation.
Graphite can also be purified using electrochemical methods, where an electric current is passed through the ore to selectively remove impurities and increase the purity of the graphite. This process, known as electrochemical exfoliation, is a relatively new technique that has shown promise in producing high-quality graphite materials for advanced applications, such as in the production of graphene, a two-dimensional material with unique electronic and mechanical properties.
Purification and refining are crucial steps in the graphite processing journey, aiming to remove impurities and enhance the quality and properties of the graphite material. These processes ensure that the final graphite product meets the desired specifications for various applications. Let's explore purification and refining in more detail:
1. Purification Techniques
Purification techniques are employed to eliminate impurities and increase the purity of the graphite concentrate obtained from beneficiation. Some common purification methods used in graphite processing include:
a. Acid Leaching: Acid leaching involves treating the graphite concentrate with acids, typically hydrochloric acid (HCl) or sulfuric acid (H2SO4), to dissolve impurities. The acid selectively reacts with minerals and elements present in the concentrate, such as metal impurities, silicates, and carbonates, leaving behind purified graphite.
b. Alkaline Cleaning: Alkaline cleaning utilizes alkaline solutions, such as sodium hydroxide (NaOH) or potassium hydroxide (KOH), to remove impurities. The alkaline solution reacts with acidic impurities, neutralizing them and facilitating their removal.
c. Thermal Treatment: Thermal treatment, also known as thermal purification or graphitization, involves subjecting the graphite concentrate to high temperatures (typically above 2,000 degrees Celsius) in an inert atmosphere. This process removes volatile impurities and transforms the graphite into highly ordered crystalline graphite with improved properties.
d. Chemical Oxidation: Chemical oxidation methods involve treating the graphite concentrate with oxidizing agents, such as potassium permanganate (KMnO4) or nitric acid (HNO3). The oxidizing agents react with organic impurities or non-graphitic carbon, oxidizing them and allowing their removal.
2. Refining Techniques
Refining techniques are employed to further enhance the quality and properties of the purified graphite. These techniques aim to optimize the particle size, shape, and surface characteristics to meet specific application requirements. Some common refining techniques in graphite processing include:
a. Grinding and Sizing: Grinding the graphite material to a specific particle size distribution is often performed to achieve the desired characteristics. Different grinding methods, such as ball milling or jet milling, can be used to control the particle size.
b. Shape Modification: Shape modification techniques, such as extrusion or compression molding, are employed to shape the graphite into various forms, including blocks, rods, powders, or flakes. These processes can help tailor the graphite material for specific applications.
c. Surface Treatment: Surface treatment methods, such as chemical treatment or coating, are applied to modify the surface properties of graphite. Surface treatments can enhance the graphite's conductivity, improve its compatibility with binders or other materials, or provide specific functionalities.
d. Intercalation: Intercalation involves inserting specific molecules or ions between the graphite layers to modify its properties. Intercalation compounds can enhance electrical conductivity, alter the interlayer spacing, or improve reactivity for certain applications.
3. Quality Control
Throughout the purification and refining processes, quality control measures are implemented to ensure that the graphite material meets the desired specifications. Various analytical techniques, including chemical analysis, thermal analysis, particle size analysis, and microscopy, are used to assess the purity, particle size distribution, crystallinity, and other relevant parameters.
Purification and refining techniques are continuously evolving to meet the increasing demands for high-purity and high-performance graphite materials. The choice of purification and refining methods depends on the initial quality of the graphite concentrate, the targeted purity level, the desired properties for specific applications, and the economic feasibility of the processes. By optimizing these steps, graphite manufacturers can produce graphite materials that meet the stringent requirements of diverse industries such as battery manufacturing, electronics, aerospace, and lubricants.
03 Applications of Graphite
BackGraphite is a versatile material with a wide range of industrial applications, thanks to its unique combination of properties, including high electrical conductivity, thermal stability, and lubricity. Some of the key applications of graphite include:
1. Batteries: Graphite is a key component in lithium-ion batteries, where it is used as an anode material to store and release electrical energy. The high conductivity and stability of graphite make it an ideal material for use in rechargeable batteries for electric vehicles, smartphones, and other portable devices.
2. Lubricants: Graphite is widely used as a lubricant in various industrial applications, thanks to its low friction and wear properties. Graphite lubricants are used in metalworking, automotive, and aerospace industries to reduce friction and wear between moving parts, leading to improved performance and longer service life.
3. Refractory materials: Graphite is also used in the production of refractory materials, which are used in high-temperature applications such as in the steel and glass industries. Graphite's high thermal conductivity and resistance to thermal shock make it an ideal material for use in refractory linings, crucibles, and molds to withstand extreme temperatures and harsh environments.
4. Carbon brushes: Graphite is used to make carbon brushes, which are essential components in electric motors and generators. Carbon brushes are used to transfer electrical current between stationary and rotating parts in machines, and graphite's high conductivity and durability make it an ideal material for this purpose.
04Conclusion
BackGraphite processing is a complex and multi-step process that involves mining, extraction, purification, and refining to produce high-quality graphite materials for various industrial applications. The versatility and unique properties of graphite make it a valuable material in a wide range of industries, from batteries and lubricants to refractory materials and carbon brushes. As demand for graphite continues to grow, advancements in processing technologies and techniques will play a crucial role in meeting the evolving needs of the industry and driving innovation in the field of graphite materials.
 +86 18716000713
+86 18716000713 xlyin@xinhaimining.net
xlyin@xinhaimining.net



 Message
Message Chat Now
Chat Now



















