What Is the Difference Between Spodumene and Lepidolite?
 Laura
Laura
 Apr 23, 2025
Apr 23, 2025
 35
35
If you want to know more details about equipment, solutions, etc, please click the button below for free consultation, or leave your requirements!
In the quest for lithium to fuel electric vehicles (EVs) and renewable energy storage, two minerals stand out: spodumene and lepidolite. While both are lithium-bearing, they differ significantly in composition, extraction methods, and economic viability. This SEO-optimized guide breaks down their differences, including the beneficiation processes that prepare them for lithium production.
01 Spodumene vs. Lepidolite: A Quick Overview
Back 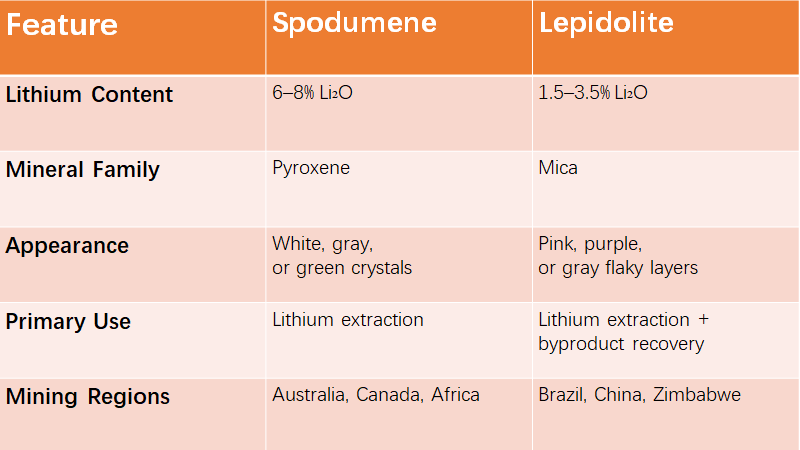
Spodumene vs. Lepidolite
02 Key Differences Explained
Back1. Lithium Content and Economic Value
Spodumene: Higher lithium concentration means less ore is needed to produce the same amount of lithium, reducing costs.
Lepidolite: Lower lithium yield requires processing more ore, but rare metal byproducts (rubidium, cesium) offset expenses.

spodumene ore
2. Physical Properties
Spodumene: Hard, dense, and resistant to weathering. Requires high-temperature roasting to become reactive.
Lepidolite: Soft, layered structure with fluorine content. Releases toxic gases during processing, complicating extraction.

lepidolite ore
3. Beneficiation Processes
Beneficiation upgrades raw ore into concentrated material. Here's how it differs:
Spodumene Beneficiation:
1). Crushing and Grinding: Ore is crushed into small pieces and ground into a powder.
2). Dense Media Separation (DMS): A dense liquid (e.g., ferrosilicon slurry) separates heavier spodumene from lighter waste minerals.
3). Magnetic Separation: Removes iron impurities using magnets.
4). Froth Flotation (Optional): Further purifies the concentrate by attaching spodumene particles to air bubbles.
More about guide to spodumene beneficiation.
Lepidolite Beneficiation:
1). Crushing and Grinding: Ore is reduced to a fine powder to liberate lithium-bearing flakes.
2). Froth Flotation: The primary method. Chemicals make lepidolite hydrophobic, allowing air bubbles to lift it to the surface as froth. Waste sinks.
3). Drying: The froth is dried into a concentrate for chemical processing.
More about guide to lepidolite beneficiation.

lithium crusher and flotation cell
4. Extraction Methods
Spodumene:
Roasting: Heated to 1,000–1,100°C to convert α-spodumene to β-spodumene, which reacts with sulfuric acid.
Acid Leaching: Produces lithium sulfate, refined into lithium carbonate or hydroxide.
Lepidolite:
Sulfuric Acid Roasting: Mixed with acid and heated to 200–300°C, releasing lithium sulfate.
Fluorine Management: Requires scrubbing systems to capture toxic fluorine gas.

Zimbabwe lithium ore processing plant site
5. Environmental Impact
Spodumene: Generates less waste per ton of lithium but involves energy-intensive roasting.
Lepidolite: Higher waste volumes and toxic byproducts demand advanced waste management.
03 Why Both Minerals Matter
BackSpodumene dominates global lithium supply due to its efficiency, especially in Australia and Africa.
Lepidolite supports regional markets (e.g., China) and diversifies supply chains, reducing geopolitical risks.
04 FAQs
BackQ: Which mineral is more abundant?
A: Spodumene is more widely mined, but lepidolite deposits are geographically diverse.
Q: Can lepidolite replace spodumene?
A: Not entirely. Its lower lithium content and complex processing make it a supplementary source.
Q: Are there greener alternatives to these minerals?
A: Direct lithium extraction (DLE) from brines and recycling could reduce reliance on both, but they remain critical for now.

Workers are checking lithium ore processing equipment
05Conclusion
BackSpodumene and lepidolite are both vital to the lithium industry, but their differences in composition, processing, and economics shape their roles. Spodumene leads in efficiency, while lepidolite offers strategic flexibility. Understanding these distinctions is key to navigating the evolving landscape of clean energy resources.
Feel free to contact us and learn more about lithium processing solutions!
 +86 18716000713
+86 18716000713 xlyin@xinhaimining.net
xlyin@xinhaimining.net



 Message
Message Chat Now
Chat Now